Abstract
Introduction: Hodgkin lymphoma (HL) is a hematologic malignancy for which frontline treatment options and outcomes have remained unchanged for several decades. HL is considered to have a favorable prognosis although failure rates after FL therapy range from 10% to 30% depending on stage. Patients who fail go on to bear the burden of additional therapy after FL. Little real-world evidence exists on the burden of disease for patients who fail FL (FLF) therapy for healthcare providers to contextualize the value of novel therapies. This study aimed to identify FLF patients and compare healthcare costs between HL patients with and without FLF.
Methods: This retrospective analysis used the MarketScan Commercial and Medicare Supplemental administrative claims databases to select adults aged >18 years with ≥ 1 inpatient or 2 outpatient claims with a HL diagnosis (ICD-9 201.xx excluding Hodgkin paragranuloma, Hodgkin sarcoma and lymphocytic-histiocytic predominance) from 1/1/2010 to 9/30/2015. Eligible patients were required to have continuous plan enrollment for > 6 months prior to the diagnosis, no prior cancer, and initiate a multi-agent regimen indicative of curative intent.
Patients were in the FLF cohort if they had one of the following failure events: initiate a new chemotherapy after discontinuing FL (gap >60 days with no chemotherapy), switch to a new treatment, or having a stem cell transplant (SCT). Patients were in FLF+XRT cohort if they also received radiation after completing FL chemotherapy. Index dates for the FLF and FLF+XRT cohorts were the earliest date of the failure events, and were randomly assigned for non-FLF patients based on distribution of intervals between FL start date and index date for the FLF cohort. They had to have > 30-day post-index continuous enrollment and followed until date of disenrollment, death, or end of the study period.
Annual healthcare costs were measured at 12-month post-index periods among patients with > 12-months post-index continuous enrollment. Costs were measured during FL and the entire follow-up period (reported as per patient per month [PPPM]).
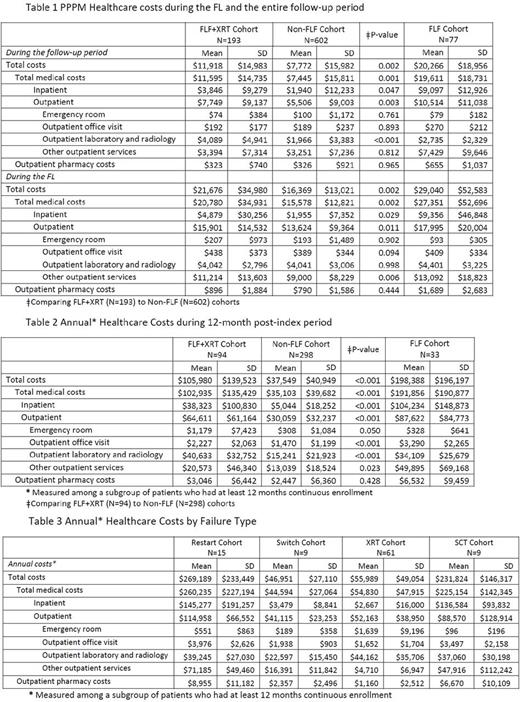
Results: 795 patients met the selection criterion (mean age 38.5 years, 47.5% female). 67.8% of patients 18-44 years old, 26.4% 45-64 years old, and 5.8% ≥65 years old. Most resided in urban areas (81.9%), and the mean pre-index Charlson Comorbidity Index score of study patients was 2.9±1.9. The two most frequent coded comorbidities during FL were symptoms involving the respiratory system (41.3%) and cardiovascular (22.9%) in both cohorts. Of the patients in the FL+XRT cohort, 60.1% received XRT after FL discontinuation, 21.8% discontinued and restarted therapy, 10.4% switched to a new therapy, and 7.8% went on to a SCT directly after FL.
The PPPM costs for FLF cohort during the follow up was $12,494 (N=77, Table 1) and the annual all-cause cost of failure was $160,839 (N=33, Table 2) higher than patients who did not fail FL. The PPPM costs in FLF+XRT cohort (N=193) was $11,918 (vs $7,772) (Table 1). The annual all-cause cost in was $68,431 (N=94) higher than patients in non-FLF cohort (Table 2). PPPM costs during FL in FLF+XRT was $21,676 (vs $16,396) (Table 1) driven by outpatient services (50.5%) and inpatient admissions (48.6%). The FLF+XRT (vs non-FLF) cohort had a significantly shorter duration of FL therapy (average of 112 vs 131 days, p<0.05).
The economic burden of failure varied by type (Table 3). The annual costs of failure due to restarting, switching, SCT, or radiation were $269,189, $46,951, $231,824, and $55,989, respectively. Similar trends in cost were observed among patients with ≥24 months post-index enrollment (n=200).
Conclusions: HL patients who fail FL represent a substantially higher economic burden compared to those without FLF, not accounting for stage at diagnosis. We did not assess the indirect costs or societal impact of FLF for the population and therefore this is an underestimate of the overall burden. More research is needed to determine optimal treatment that could reduce the risk of progression, the need for treatment after completion of FL and enhance long-term clinical and economic outcomes.
Straus: Received consulting fee from Seattle Genetics for involvement in the research: Consultancy. Bonafede: Employee of Truven Health Analytics, an IBM Company, that received a research contract to conduct this study with and on behalf of Seattle Genetics, Inc: Employment. Feliciano: Seattle Genetics: Employment, Equity Ownership. Cai: Truven Health Analytics: Employment, Other: I am an employee of Truven Health Analytics, an IBM company which received funding from Seattle Genetics to conduct this study. Noxon: Truven Health Analytics, an IBM company which received project funding from Global Blood Therapeutics: Employment. Princic: Truven Health Analytics, IBM Company: Employment. Josephson: Seattle Genetics: Employment, Equity Ownership. Richhariya: Seattle Genetics: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal